Stoichiometry Sheet 6 Answers - • balancing equations • writing a chemical equation • stoichiometry practice: Stoichiometry with solutions name _____ 1. Base your answer to the following question on the information below. Chapter 6 balancing and stoichiometry worksheet and key topics: From the amount of nitric acid given in part a, how many moles of nitrogen monoxide will be made? The decomposition of sodium azide, nan3(s), is used to inflate. 2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 8) if i do this reaction with 35 grams of c 6 h 10 and 45.
From the amount of nitric acid given in part a, how many moles of nitrogen monoxide will be made? Chapter 6 balancing and stoichiometry worksheet and key topics: The decomposition of sodium azide, nan3(s), is used to inflate. 2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 8) if i do this reaction with 35 grams of c 6 h 10 and 45. • balancing equations • writing a chemical equation • stoichiometry practice: Base your answer to the following question on the information below. Stoichiometry with solutions name _____ 1.
2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 8) if i do this reaction with 35 grams of c 6 h 10 and 45. The decomposition of sodium azide, nan3(s), is used to inflate. Stoichiometry with solutions name _____ 1. From the amount of nitric acid given in part a, how many moles of nitrogen monoxide will be made? Chapter 6 balancing and stoichiometry worksheet and key topics: • balancing equations • writing a chemical equation • stoichiometry practice: Base your answer to the following question on the information below.
Free Printable Stoichiometry Worksheets, 60 OFF
From the amount of nitric acid given in part a, how many moles of nitrogen monoxide will be made? Stoichiometry with solutions name _____ 1. 2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 8) if i do this reaction with 35 grams of c 6 h 10 and 45. Base your.
Stoichiometry Practice Worksheets Answers Chemistry
2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 8) if i do this reaction with 35 grams of c 6 h 10 and 45. • balancing equations • writing a chemical equation • stoichiometry practice: From the amount of nitric acid given in part a, how many moles of nitrogen monoxide.
Stoich w ssr_and_dr with key PDF Worksheets Library
The decomposition of sodium azide, nan3(s), is used to inflate. Base your answer to the following question on the information below. From the amount of nitric acid given in part a, how many moles of nitrogen monoxide will be made? 2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 8) if i.
Mole Calculations 10th Grade Quiz Quizizz
The decomposition of sodium azide, nan3(s), is used to inflate. Stoichiometry with solutions name _____ 1. • balancing equations • writing a chemical equation • stoichiometry practice: Chapter 6 balancing and stoichiometry worksheet and key topics: From the amount of nitric acid given in part a, how many moles of nitrogen monoxide will be made?
Stoichiometry Worksheets With Answers
• balancing equations • writing a chemical equation • stoichiometry practice: Base your answer to the following question on the information below. 2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 8) if i do this reaction with 35 grams of c 6 h 10 and 45. Chapter 6 balancing and stoichiometry.
Stoichiometry Practice Worksheets Answers Chemistry
From the amount of nitric acid given in part a, how many moles of nitrogen monoxide will be made? Stoichiometry with solutions name _____ 1. Chapter 6 balancing and stoichiometry worksheet and key topics: • balancing equations • writing a chemical equation • stoichiometry practice: Base your answer to the following question on the information below.
SOLUTION Chemistry stoichiometry practice problems with answers
Stoichiometry with solutions name _____ 1. From the amount of nitric acid given in part a, how many moles of nitrogen monoxide will be made? • balancing equations • writing a chemical equation • stoichiometry practice: The decomposition of sodium azide, nan3(s), is used to inflate. 2 c 6 h 10 + 17 o 2 12 co 2 + 10.
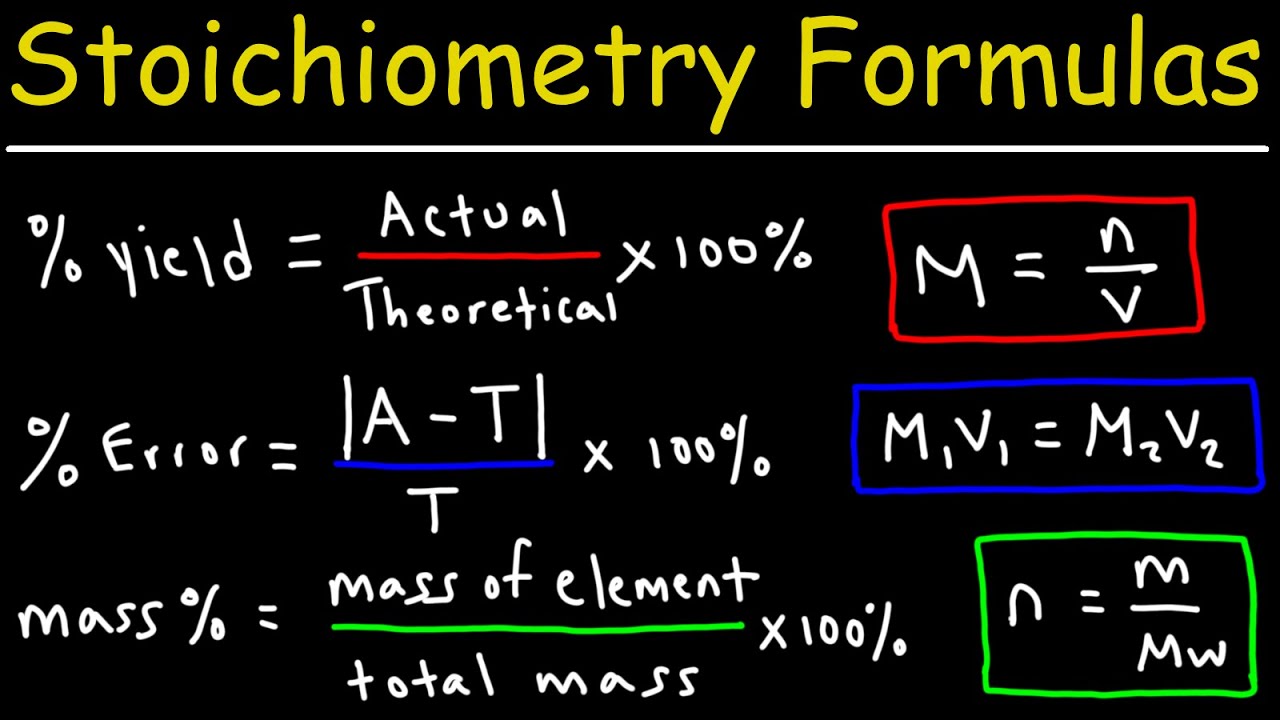
Stoichiometry Formulas and Equations College Chemistry YouTube
Base your answer to the following question on the information below. Stoichiometry with solutions name _____ 1. The decomposition of sodium azide, nan3(s), is used to inflate. From the amount of nitric acid given in part a, how many moles of nitrogen monoxide will be made? • balancing equations • writing a chemical equation • stoichiometry practice:
Stoichiometry Problems Worksheets
• balancing equations • writing a chemical equation • stoichiometry practice: 2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 8) if i do this reaction with 35 grams of c 6 h 10 and 45. From the amount of nitric acid given in part a, how many moles of nitrogen monoxide.
Worksheet For Basic Stoichiometry Printable And Enjoyable Learning
2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 8) if i do this reaction with 35 grams of c 6 h 10 and 45. Chapter 6 balancing and stoichiometry worksheet and key topics: From the amount of nitric acid given in part a, how many moles of nitrogen monoxide will be.
• Balancing Equations • Writing A Chemical Equation • Stoichiometry Practice:
2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 8) if i do this reaction with 35 grams of c 6 h 10 and 45. From the amount of nitric acid given in part a, how many moles of nitrogen monoxide will be made? Base your answer to the following question on the information below. Chapter 6 balancing and stoichiometry worksheet and key topics:
Stoichiometry With Solutions Name _____ 1.
The decomposition of sodium azide, nan3(s), is used to inflate.