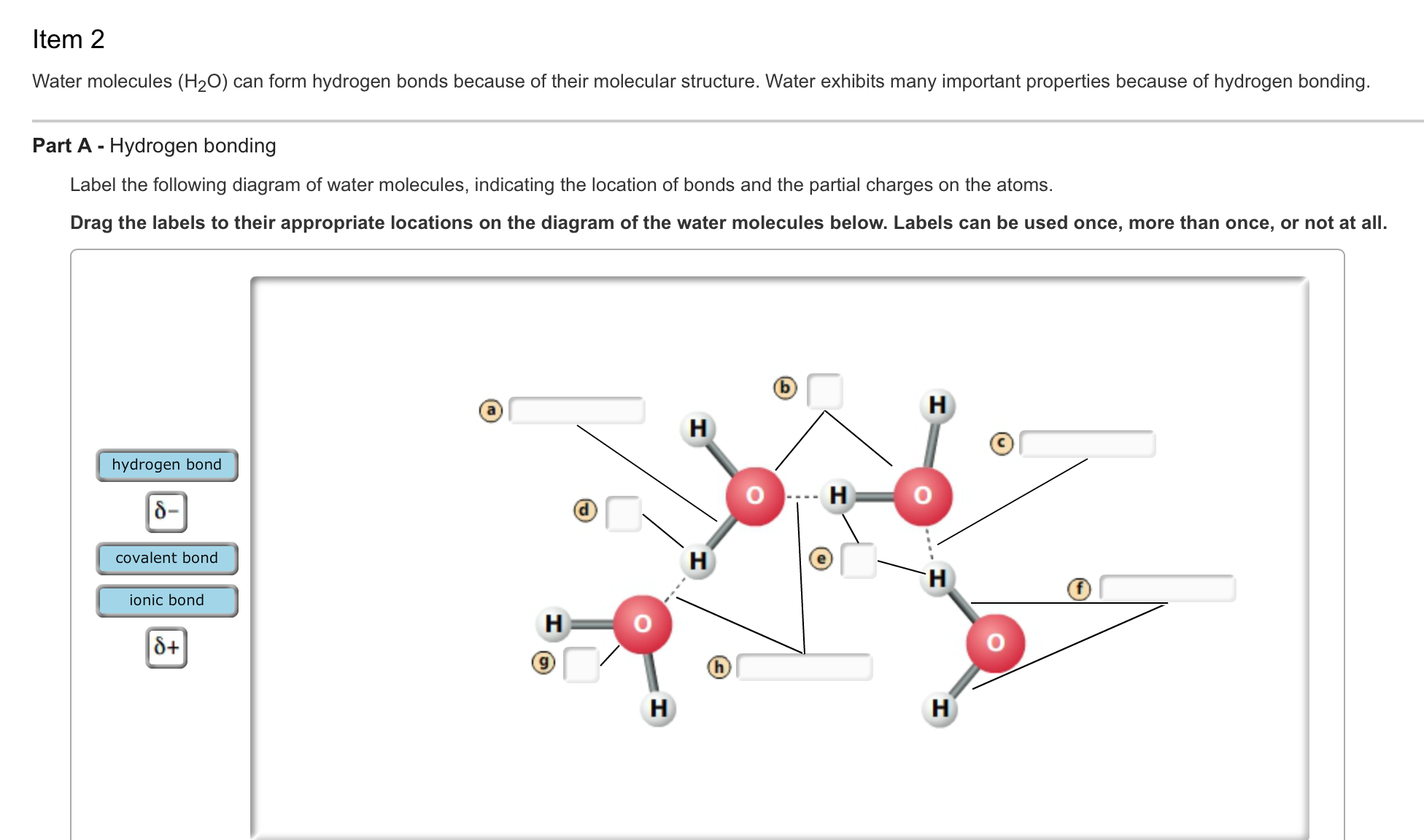
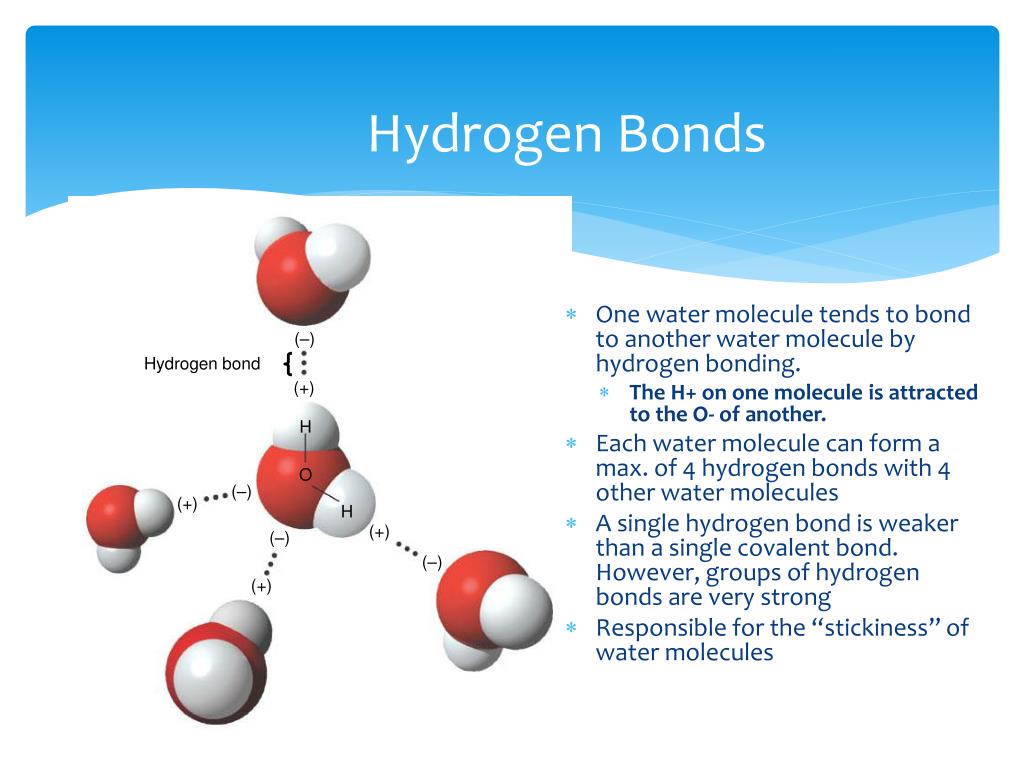
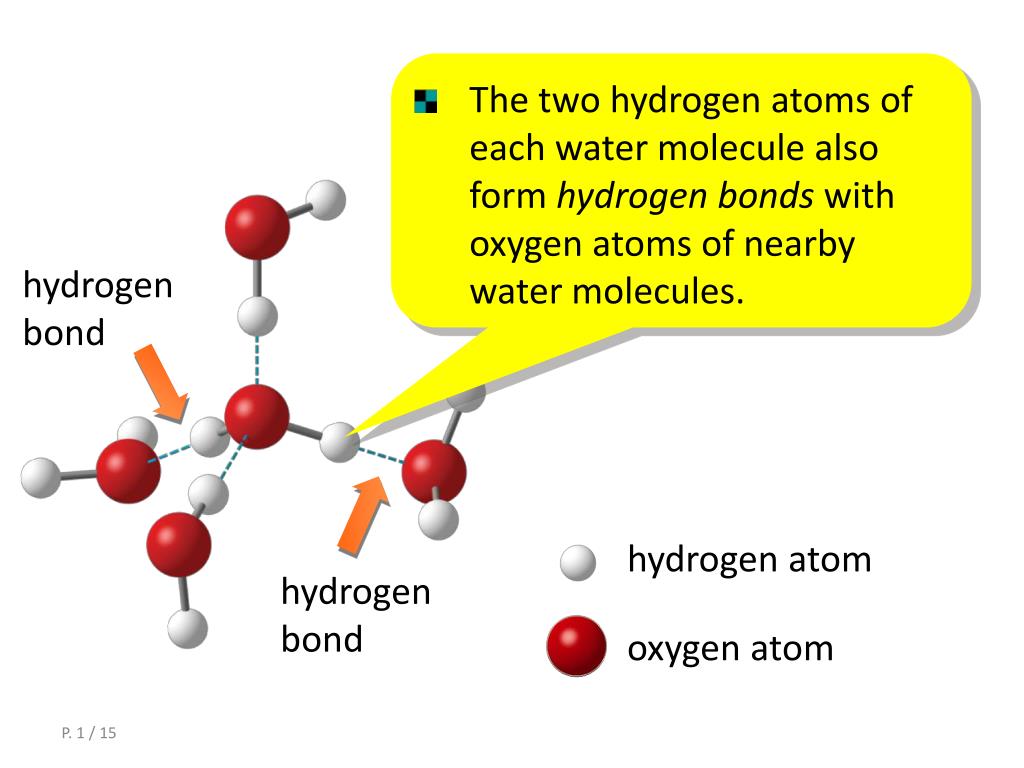
How Do Hydrogen Bonds Form Between Water Molecules - Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its.
The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of. Identify three special properties of water that make it unusual for a molecule of its.
The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of.
Water Molecules Hydrogen Bonds
Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of.
Solved Water molecules (H_2O) can form hydrogen bonds
Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its.
Water Molecules Hydrogen Bonds
The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of. Identify three special properties of water that make it unusual for a molecule of its.
Water Molecules And Hydrogen Bonding Diagram
The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of.
Draw A Hydrogen Bond Between Two Water Molecules
Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of.
Are Hydrogen Bonds The Strongest Interactions Between Molecules?
The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of.
Water Molecules Hydrogen Bonds
Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of.
Diagram of hydrogen bonding between two water molecules Download
The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of.
Draw A Diagram Of Water Molecules Labeling The Hydrogen Bond And
Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule.
Hydrogen Bonds Form Between Water Molecules When The Partially Positive End Of.
Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule.